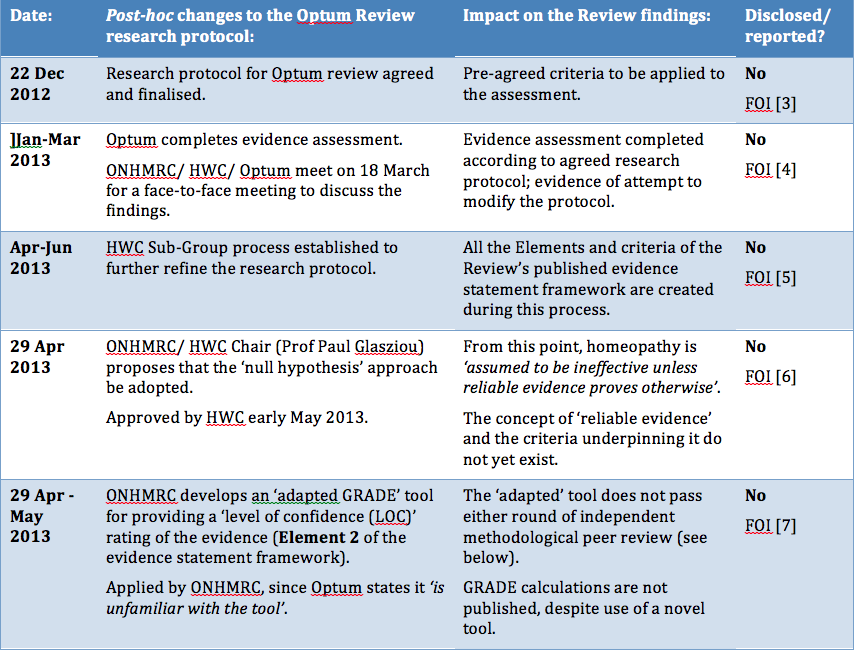
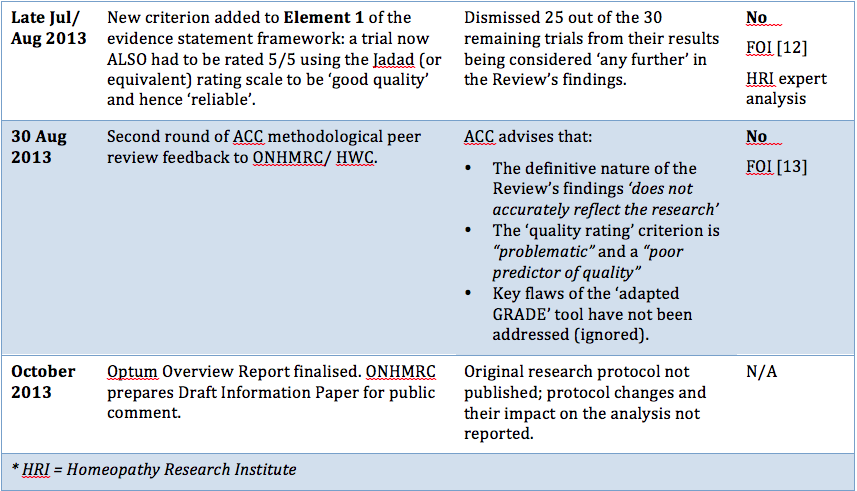
Procedural irregularities
|
"Research fraud and administrative misconduct undermines the public’s trust in science and results in loss of trust in public institutions. "... it is important that the methods to be used should be established and documented in advance. Publication of a protocol for a review prior to knowledge of the available studies reduces the impact of review authors’ biases, promotes transparency of methods and processes, reduces the potential for duplication, and allows peer review of the planned methods (Light 1984)." "It is important, however, that changes in the protocol should not be made on the basis of how they affect the outcome of the research study. Post hoc decisions made when the impact on the results of the research is known, such as excluding selected studies from a systematic review, are highly susceptible to bias and should be avoided." "I am concerned that no homeopathic expert was appointed to the NHMRC Review Panel. I cannot imagine this being agreed in oncology, orthopaedics or other disciplines." |